Dairy processors can rely on Eurotherm for the digitization of pasteurization temperature and process data
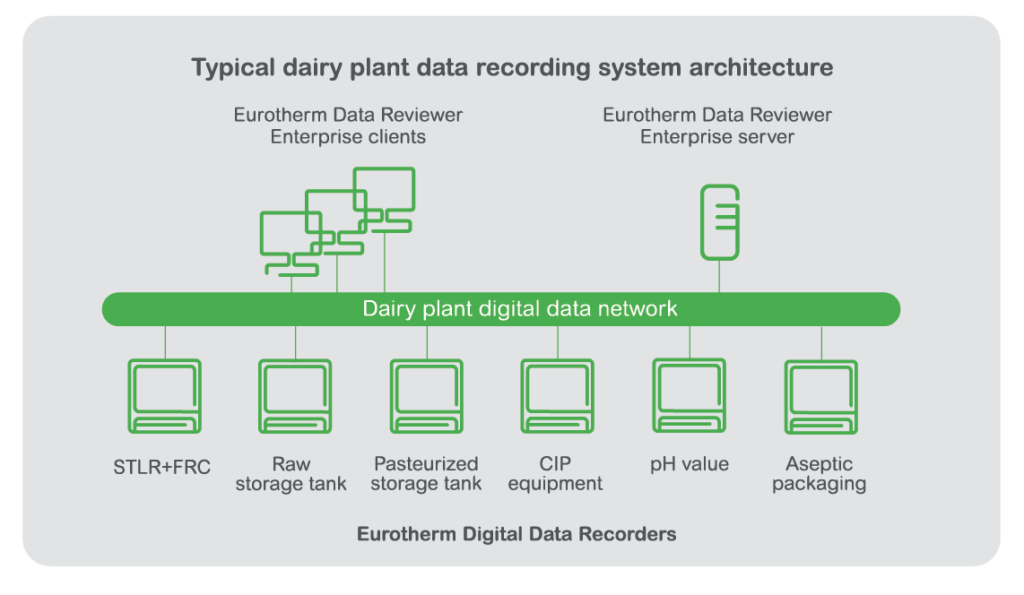
The pasteurization of milk products is a heavily regulated process and is critical to public health and safety. Pasteurization process regulations such as the U.S. Department of Health and Human Services, Public Health Service and FDA Grade “A” Pasteurized Milk Ordinance (PMO), have specific data requirements for milk pasteurization areas and equipment. These include, safety thermal limit recorders (STLR), flow recorder/controllers (FRC), storage tanks, clean-in-place (CIP) equipment and aseptic packaging. Whether your plant utilizes HTST (high temperature short time) pasteurization methods or produces UHT milk, Eurotherm has the solution to fit your needs.
Data recorders/controllers used for milk pasteurization play an important role in helping to ensure every particle of milk product is exposed to the appropriate temperature for the appropriate time.
Eurotherm digital data recorders offer a range of features and solutions to help dairy processors meet the data recording and control requirements of the PMO and other regional regulations.
Future-proof your business operations by expanding process digitalization throughout the dairy plant
Eurotherm helps to bring improved efficiency and sustainability to dairy plants by enabling digitization of the data collection for milk pasteurization and other dairy plant processes. The digital recording of contextual metadata helps to improve data integrity and increase the trustworthiness of dairy plant process data.
Data management for Food & Beverage >
Compliance
Tamper resistant features in Eurotherm digital data recorders help to meet:
- FDA Grade “A” Pasteurized Milk Ordinance (PMO) criteria for electronic data recording (M-b-355)
- FDA Grade “A” Pasteurized Milk Ordinance (PMO), VI. CRITERIA FOR THE EVALUATION OF COMPUTERIZED SYSTEMS FOR GRADE “A” PUBLIC HEALTH CONTROLS. 2019 Revision
- FDA 21 CFR Part 11 requirements for secure Electronic Records and Electronic Signatures
- Data Integrity ALCOA+ principles
Digitalization
- Increase the reliability of dairy plant data and processes by moving away from paper data recording to digital solutions
- Efficiencies
- Recognize cost savings associated with a movement from paper to digital data recording
- Sustainability
- Future-proof your business by reducing risk and increasing manufacturing intelligence
- Key dairy plant performance drivers
- Enhanced food safety
- Meet regulatory compliance
- Reduce business cost by migrating from paper to digital data recording
- Increase manufacturing intelligence to achieve higher quality and efficiency
- Enable higher levels of data integrity
- Reduce business risk with enhanced sustainability and security
- Improve product traceability
Need to meet public health control recording requirements for PMO criteria?
Eurotherm standardized STLR/FRC solutions can drive improved efficiencies while simplifying compliance to regulatory requirements
Regulation and Guidance
In the US dairy industry, the PMO requires data recorders to be configured to prevent any milk product being pasteurized from falling below a specific pasteurization temperature. Regulations also state that data recorders in pasteurization systems with variable speed timing pumps must ensure that pasteurized product flow does not exceed machine design.
Similar regulations exist in other regions around the world. For example:
- Canadian Food Inspection System (CFIS) National Dairy Code, Production and Processing Requirements, Parts I, II, and III
- European Union Commission Regulation – no. 605/2010
- Food Safety and Standards Authority of India (FSSAI) – Food Safety Management Systems
- Food Standards Australia and New Zealand – Primary Production and Processing Standard for Dairy Products
Best practice guidance advises the use of digital data recording solutions, which should comply with the requirements of the Grade “A” Pasteurized Milk Ordinance (PMO), FDA 21 CFR Part 11 standard for Electronic Records and Electronic Signatures, and the ALCOA+ data integrity principles.
Eurotherm 6100A/6180A standardized STLR/FRC solutions
As part of our digital engineered solutions (DES), we have developed standardized STLR/FRC solutions for Safety Thermal Limit Recorder (STLR) and Flow Recorder Controller (FRC) applications.
- Pre-configured to help meet the data recording and flow control requirements of the FDA PMO, FDA 21 CFR Part 11, and ALCOA+ data integrity principles
- Allow customization to include machine builder/end user intellectual property
6100A/6180A Standardized STLR+FRC Solutions >

Eurotherm quick links:
Calculate savings with digital recording >
Pasteurization solution note >
PMO compliance to public health control criteria white paper >
PMO compliance for the use of electronic data recorders white paper >
6180A STLR+FRC solution user guide >
Food and Beverage solutions >































